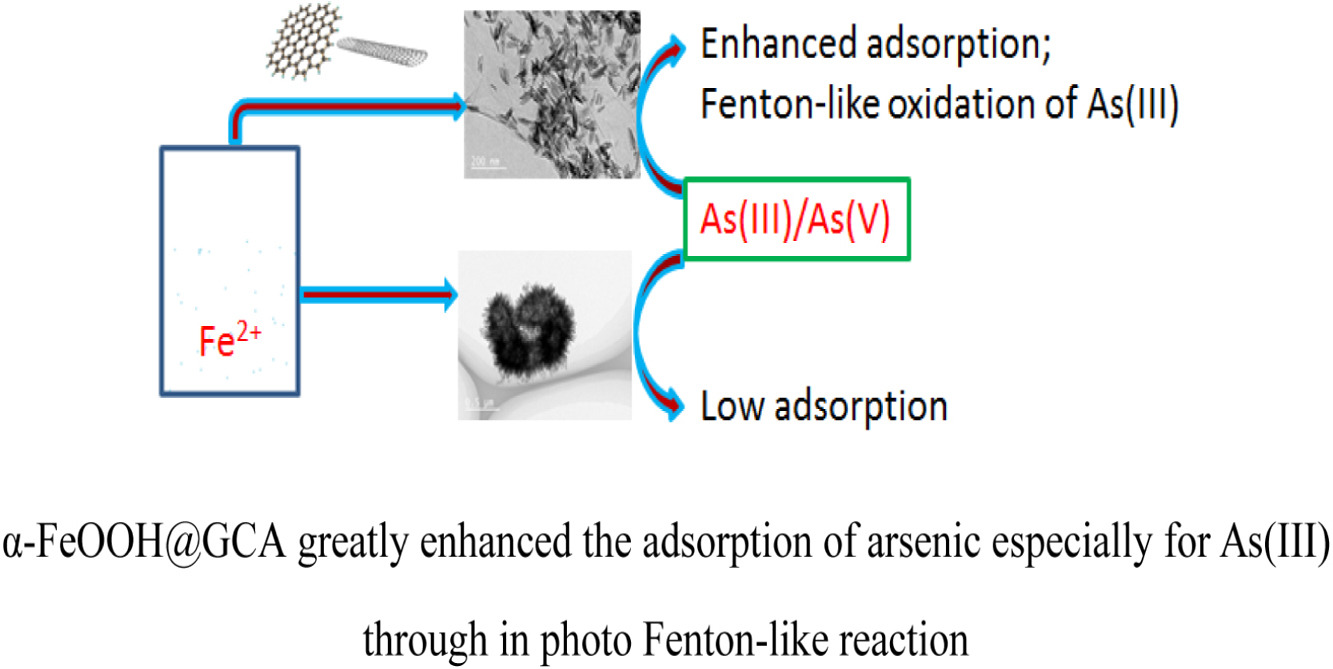
Arsenite (As(III)) is less efficient removal than arsenate (As(V)) by adsorption process. Integration of pre-oxidation to convert As(III) to As(V) with simultaneous in-situ adsorption of formed As(V) would be highly desirable. In this work, a novel three-dimensional (3D) bifunctional α-FeOOH@GCA nanocomposite prepared through goethite anchored graphene oxide nanosheet (GO)-carbon nanotubes (CNTs) as supporting materials for arsenic removal. The adsorption process of arsenic species (As(III)/As(V))onto α-FeOOH@GCA nanocomposite surface is independent of pHs, however, the arsenic adsorption edges migrate along with the competition between arsenic species due to the same adsorption sites to form inner-sphere complexes. α-FeOOH@GCA shows remarkably enhanced iron mass normalized adsorption capacity of 236.50 mg-As(III)/g-Fe or 450.72 mg-As(V)/g-Fe which were about 6 or 10 fold that of pristine α-FeOOH, respectively. GO-CNTs play a very important role in enhancing arsenic adsorption capacity by α-FeOOH through forming unique interfacial structures and measured by X-ray photoelectron analysis (XPS) study: (1) greatly miniaturize the size of pristine α-FeOOH and uniformly dispersed onto GO-CNTs surface; (2) increase the adsorption surface area of pristine α-FeOOH nanoparticles. α-FeOOH@GCA nanocomposite also exhibits superior high selectivity/affinity for As(V) to As(III) and facilitates the pre-oxidation of As(III) and simultaneous As(V) adsorption. In-situ photo Fenton-like oxidation increased the adsorption efficiency of As(III) to 80% comparing with direct adsorption ratio of As(III) (∼36%). The complete regeneration of arsenic adsorbed α-FeOOH@GCA proves the durability of this efficient bifunctional composite for synergistically realizing oxidation–adsorption for arsenic removal in water system, especially for more mobile As(III) removal. © 2021 Elsevier B.V.
2021
A bifunctional α-FeOOH@GCA nanocomposite for enhanced adsorption of arsenic and photo Fenton-like catalytic conversion of As(III)
- Environmental Technology and Innovation
Pervez M.N., Fu D., Wang X., Bao Q., Yu T., Naddeo V., Tian H., Cao C., Zhao Y.
Abstract
Document Type:
Share:
Graphical Abstract